
Trojan Technologies has the largest installed base of applications in the Life Sciences industry, meeting the demands of USP and WFI applications.
For over 70 years, Aquafine UV systems have been successfully serving the diverse Life Sciences industry. Aquafine UV systems are reliable, deliver consistent performance, and have become the brand of choice of WFI (Water-For-Injection) & USP (United States Pharmacopoeia) systems. Aquafine systems are found in both the pre-treatment and process areas of the water system. While commonly found in microbiological inactivation and ozone reduction applications, our low-pressure (LP) and low-pressure high-output (LPHO) systems also can be found in TOC (total organic carbon) reduction and chlorine/chloramine reduction applications.
Trojan Technologies offers validated systems, providing UV lamp and NIST traceable UV sensor certificates. All systems comply with cGMP and FDA requirements, and sanitary connections conform to DIN and USDA 3A standards. Select models carry the marks of cULus, CE and ANSI/NSF and can be mounted horizontally or vertically, or in skid mounted systems, maximizing installation flexibility and preserving floor space.
With Life Sciences UV system designs unparalleled in performance, Trojan Technologies is committed to providing superior quality and the latest advancements in UV technology.
UV technology for water treatment has several inherent advantages. Nothing is added to the water stream by, such as color, odor, or by-products. UV technology is a fast, efficient, and cost-effective solution that can inactivate microorganisms as well as reduce chlorine/chloramine and ozone in the water. UV can also be utilized to product
✓ Contact time required is minimal
✓ Fast and efficient treatment
✓ Compact system footprint
✓ No impact on taste, odor, or color
This is the most common application of UV light in water treatment. A pharmaceutical water system could have several locations where UV equipment would be installed, including post-carbon filter and pre-RO (reverse osmosis). When installed downstream of the carbon bed and/or directly upstream of the RO unit, a UV system can significantly reduce the microbial counts by inactivating the microorganisms present in the influent stream. Inactivation is also recommended for the process distribution loop and pre storage tank.
UV using low-pressure lamps is a highly effective, versatile, and reliable method to address chloramine reduction. Studies have demonstrated conclusively that chloramine residuals up to 4 ppm can be successfully reduced to by the application of UV light. The breakdown products from treating monochloramine with UV are primarily non-hazardous ionic species and subsequently removed by the downstream RO system. At typical pH and dissolved oxygen levels in municipal treated waters, ammonia formation is negligible.
Ozone is commonly used in the pre-treatment area of a water system, as well as for sanitizing process and recirculating systems. Prior to the point-of-use, the residual ozone needs to be reduced to ensure the process water is not compromised. Because it is a fast-acting treatment, UV technology is the preferred method for this application. A properly sized UV unit can reduce the ozone to non-detectable limits, ensuring the integrity of the process and the product. A dosage of 90 mJ/cm² is recommended for reduction of ozone residuals of 1.0ppm.
![]()
WFI is produced by a distillation or reverse osmosis (RO) process that removes chemicals and microorganisms from water. High intensity UV lamps are proven to reduce endotoxins, microbial loads, TOC, residual ozone, and chloramines. WFI that features UV inactivation affords several advantages over auxiliary purification methods including efficacy, cost, and customization of configuration.
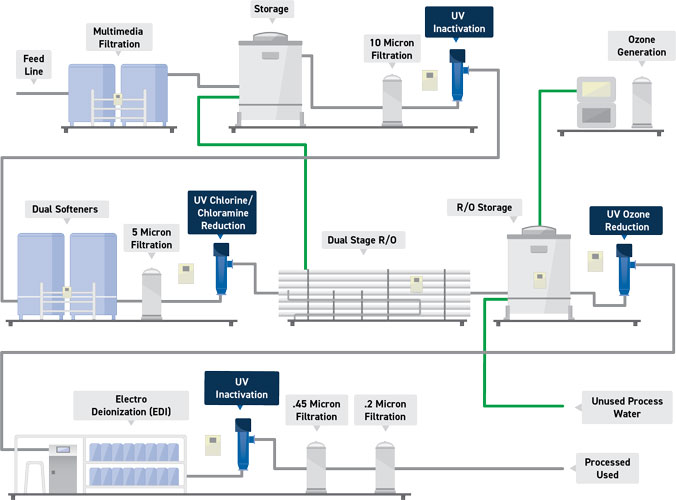
A Trojan Technologies water treatment system produces WFI through reverse osmosis and UV inactivation. The water enters a WFI skid and is pretreated to remove particulates with multimedia filtration, a water softener, and 5 micron filtration stage. The water’s chlorine and chloramines are then UV inactivated before undergoing reverse osmosis to remove organics, colloids, and microbes. Water is then stored and further purified in an ozonated storage tank before passing through a UV light chamber for ozone removal. Refinement continues with electro deionization and additional UV Inactivation before passing through .45 micron filtration and .2 micron filtration. The end result is ideally pure WFI that is compliant and ready for multiple points of use.
For applications that rely on high quality water of the utmost purity, WFI with UV inactivation is superior to its alternatives due to the meticulous production processes and the high level of regulatory oversight involved, which permit only the lowest amounts of contaminants. That WFI meets and exceeds these standards is why pharmacopeias like US Food and Drug Administration (FDA), the World Health Organization (WHO), European Pharmacopoeia (EP), and United States Pharmacopoeia (USP) deem it acceptable for use in the production of injectable drugs and other pharmaceutical products.
Contact us today to speak to an expert.
*Specific UV systems manufactured by Trojan Technologies have been validated through microbial testing. Through this testing, performance data has been generated for UV dose delivery to inactivate Escherichia coli (E. coli), fecal coliform, Poliovirus, Cryptosporidium, Giardia, and Adenovirus. For a detailed list of UV systems and target organisms, visit www.trojantechnologies.com/en/support/treatment-claims