Ultraviolet (UV) light is a form of light that is invisible to the human eye. It occupies the portion of the electromagnetic spectrum between X-rays and visible light.
A unique characteristic of UV light is that a specific range of its wavelengths, those between 200 and 300 nanometers (billionths of a meter), they are capable of inactivating microorganisms such as Cryptosporidium and Giardia. This capability has allowed widespread adoption of UV light as a highly effective way to treat wastewater and drinking water.
A UV lamp is quite different than your standard incandescent light bulb. Yes, electricity is still passed through a tungsten filament which heats up, but that energy “excites” a very small amount of mercury vapor contained in the lamp. It is the mercury vapor that glows and emits the UV light.
Electricity is passed through a tungsten filament which heats up, and that energy “excites” a very small amount of mercury vapor contained in the lamp.
In water treatment applications, UV light provides rapid, effective inactivation of microorganisms through a physical process. When microorganisms are exposed to wavelengths of UV light, they are instantaneously rendered incapable of reproducing.
Microorganisms are inactivated by UV light as a result of damage to nucleic acids. The high energy associated with short wavelength UV energy, primarily at 254 nm, is absorbed by cellular RNA and DNA. This absorption of UV energy forms new bonds between adjacent nucleotides, creating double bonds or dimers. Dimerization of adjacent molecules, particularly thymine, is the most common photochemical damage. Formation of numerous thymine dimers in the DNA of microorganisms prevents replication.
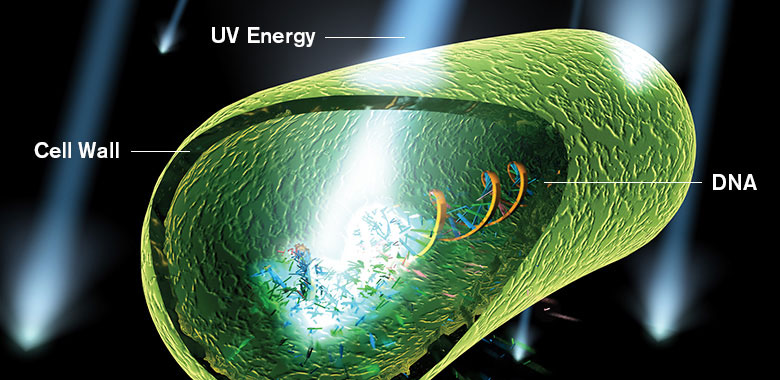
Rendering of UV energy damaging a microorganism's DNA.
UV light has demonstrated efficacy against organisms, including those responsible for cholera, polio, typhoid, hepatitis and other diseases.
A significant body of scientific research has proven UV light’s ability to inactivate an extensive list of microorganisms. UV offers a key advantage due to its ability to inactivate certain chlorine-resistant microorganisms – most notably Cryptosporidium and Giardia.
The release of these microorganisms into receiving lakes and rivers by wastewater facilities utilizing chlorine increases the potential of contamination in communities that rely on these same bodies of water for their drinking water source and recreational use. Drinking water treatment plants can benefit by using UV since it can easily inactivate chlorine-resistant microorganisms, while reducing chlorine usage and by-product formation.
Key advantages:
Requires no transportation, storage or handling of chemicals
Does not create carcinogenic by-products
Highly effective at inactivating a broad range of microorganisms – including chlorine-resistant Cryptosporidium and Giardia
Can be used in conjunction with an oxidant (e.g., hydrogen peroxide) to break down and reduce chemical contaminants in water
The effects of UV are directly related to the dose of UV energy absorbed by a microorganism. UV dose is a product of UV intensity and residence time and the required treatment limit/log reduction will dictate the required UV dose. UV dose is typically expressed in mJ/cm2 or uWs/cm2.
The residence time of the UV system is determined by the chamber design and the flow rate of the water. The intensity is affected by the equipment parameters (such as lamp type, lamp arrangement, etc.) and water quality parameters (such as UV transmittance, TSS, etc.). Unlike other treatments, UV treatment is not affected by the temperature or pH of the water.
To accurately determine the dose of the UV system for a given flow rate, bioassay validation must be conducted to take into account all the variables that can affect the UV intensity and residence time.
Municipal
Industrial
Have questions? Call us at 1 (888) 220-6118 or complete the form below.